
Mastering eCTD for Africa’s Evolving Regulatory Landscape
The electronic Common Technical Document (eCTD) has become the global standard for regulatory submissions, streamlining how applications are prepared, validated, and reviewed. As more African regulatory authorities move towards digital submission systems, it is essential for pharmaceutical professionals across the continent to be prepared. At the AfriSummit eCTD Training, you will gain practical, hands-on expertise in processing eCTD submissions tailored to the African regulatory environment, while also aligning with international standards.
Why Attend?
- Africa-Focused Training – Understand how eCTD adoption is evolving across the continent, the challenges faced by companies, and practical solutions for successful submissions.
- Hands-On Learning – Work through real-world case studies, role-play exercises, and group activities simulating eCTD submission scenarios.
- Expert Guidance – Learn directly from EXTEDO’s Product Manager, Maren Mueller, who brings years of experience in supporting both health authorities and industry with eCTD compliant software solutions and regulatory expertise worldwide.
- Global Perspective – Stay informed on international trends shaping eCTD adoption and discover how Africa can harmonize its regulatory practices with established frameworks in Europe, GCC, Asia, and beyond.
- Boost Your Skills & Career – Whether you are new to eCTD or are already familiar, this training equips you with the confidence and expertise to drive digital transformation in regulatory affairs.


What Will You Learn?
- Fundamentals of eCTD structure and submission requirements
- Step-by-step guidance on compiling, validating, (re-)viewing, and submitting dossiers
- Best practices to ensure compliance with regulatory authority expectations
- Common challenges in eCTD implementation – and how to overcome them
- Insights into global regulatory frameworks and their relevance to Africa
Training Format
- Pre-Learning Access – Once registered, participants will receive access to an exclusive e-Learning portal to prepare ahead of the training.
- Interactive Full-Day Workshop – Practical group exercises, simulations, and guided sessions with the trainer.
- Mandatory Requirement: Delegates must bring their laptops for the training to fully participate in the hands-on sessions.


MAREN MUELLER
Product Manager – EXTEDO
Maren joined EXTEDO, a cormeo brand, in 2011 and started in the Customer Care department to support with regulatory knowledge and software training. After a while, moved as a Senior Business Consultant to the Regulatory Competence Center, where she worked closely with many health authorities and pharmaceutical companies on different projects.
Since 2017, she is a member of the Product Management Team, responsible for several Apps in the Submission and Document Management Hub. As a Product Manager, she still works very closely with different customers around the globe.
With over 14 years of experience in regulatory consulting and product management, Maren brings practical expertise and deep industry insights.

cormeo unites the expertise of Docuvera, EXTEDO, Rote Liste, and medicines.ie to provide a seamless end-to-end approach to creating, managing, and distributing regulated information in the life sciences sector.
Together, we build the trusted bridge between industry, authorities, healthcare professionals, and patients, facilitating the innovative and compliant exchange of regulated information in life sciences to improve patient health.
As part of cormeo, EXTEDO is a leading provider of life science data and document management software and services for industry and health authorities worldwide. The EXTEDO pulse suite helps with automating and accelerating business processes during every step of the pharmaceutical product lifecycle. EXTEDO’s Effortless Compliance™ solutions and expert consulting services ensure compliance with global regulations, saving you time, reducing costs, and minimizing errors.
As the trusted provider of software solutions for the Egyptian Health Authority (EDA), EXTEDO supported the agency in implementing the eCTD standard in Egypt.
Who Should Attend?
This training is ideal for:
- Regulatory Affairs professionals
- Submission Managers & Specialists
- Quality & Compliance Officers
- Professionals involved in dossier preparation and electronic submissions
- Representatives from pharmaceutical companies, biotech firms, CROs, and regulatory authorities

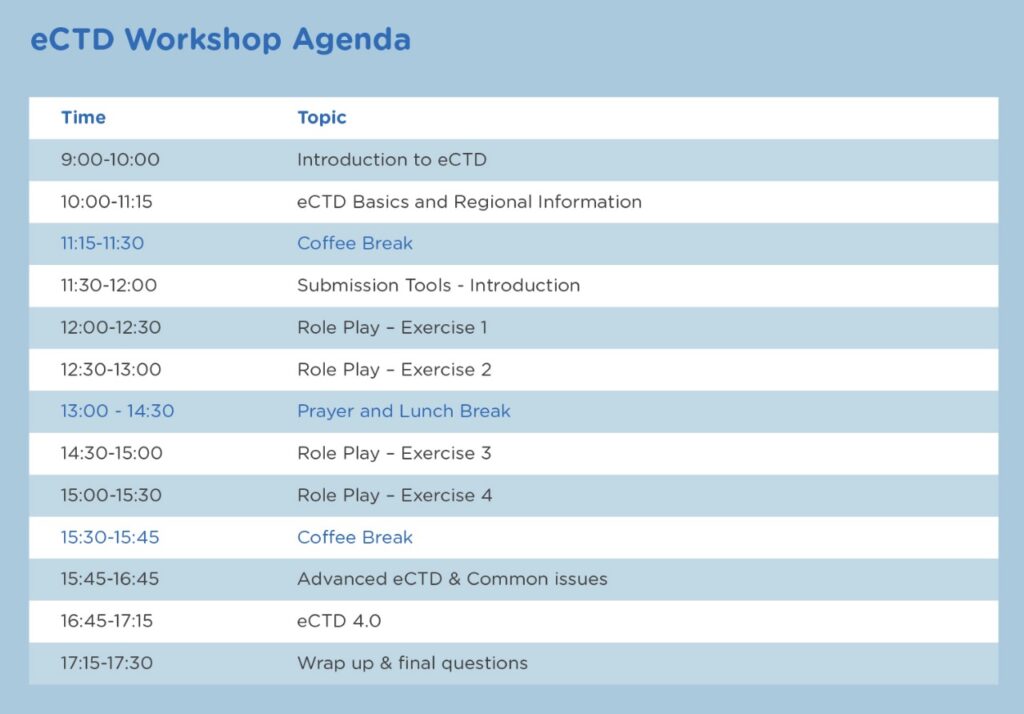
Agenda :
Reserve Your Seat Today
The AfriSummit eCTD Training is your opportunity to stay ahead of regulatory digitalization and ensure your organization is ready for the future of pharmaceutical submissions.
Date: TBC
Venue: TBC






